Author: Patricia Buškulić, mag.ing.geol.
Isotopes are atoms of the same chemical element that have the same number of protons but different number of neutrons in their nucleus. Take the same position in the table of elements, have the same chemical properties but different mass number. There are stable and unstable, i.e. radioactive isotopes (radionuclides). Oxygen, for example, has three stable isotopes: 16O, with 8 protons and 8 neutrons; 17O, with 8 protons and 9 neutrons; and 18O, with 8 protons and 10 neutrons (Figure 1). In nature, the most abundant oxygen isotope is oxygen-16 (99.76 %), afterwards oxygen-18 (0.2 %) and then oxygen-17 (0.038 %), while the most abundant hydrogen isotope is hydrogen-1 (99.985 %) and then hydrogen-2 (0.015 %). The most relevant isotopes related to hydrology are oxygen-18 and hydrogen-2 (or deuterium, D) ( Mook 2001). Oxygen and hydrogen isotopes form a different types of stable water molecules. For every 10000 water molecules we would find 9977 molecules H216O, 3 molecules H217O and 20 molecules H218O (Figure 2). The rate at which heavy and light stable isotopes react during physical or chemical reactions differs due to mass differences. The heavy isotopes react more slowly than the light isotopes which leads to isotopic separation or fractionation (Clark, 2015).

Figure 1. Oxygen Stable Isotopes (source: Climate Science Investigation. URL: http://www.ces.fau.edu/nasa/module-3/how-is-temperature-measured/isotopes.php)

Figure 2. Stable Water Isotopes (source: Program on Climate Change. URL: https://uwpcc.ocean.washington.edu/file/Water_Isotopes)
Stable isotope concentrations are measured as a ratio of the rare to the abundant isotope. The most commonly used is delta (δ) notation which indicates the difference in ratio between the sample and a known reference expressed in permil units (Clark, 2015). The isotopic composition of water is expressed in comparison with the average isotopic composition of a seawater sample. The standard for water sample is determined by an international agreement and is called Standard Mean Ocean Water (SMOW). SMOW has recently run out and has been replaced with Vienna Standard Mean Ocean Water (VSMOW) developed by the International Atomic Energy Agency (IAEA) from distilled seawater that was modified to have an isotopic composition close to SMOW.
In nature, ratio differences occur in the aqueous medium due to phase transitions. The isotopic separation (fractionation) of stable isotopes of oxygen and hydrogen mostly depends on the phase transition temperature, pressure and initial isotopic composition of water. For example, during evaporation light isotopes evaporate more readily so the water remains enriched with heavy isotopes, while during condensation heavy water isotopes rain and snow more readily (Figure 3). The isotopic composition of precipitation decreases with distance from the coastline, because the more clouds travel to the continent and the more precipitation falls along that path, the precipitation will have more light and less heavy isotopes. Factors influencing the spatial and temporal distribution of water isotopic composition are shown in Figure 4.
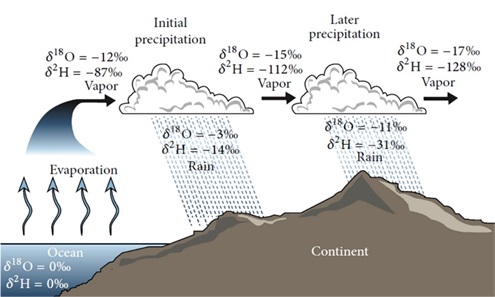
Figure 3. Schematic review of isotopic fractionation within the hydrological cycle (Xi, 2014)
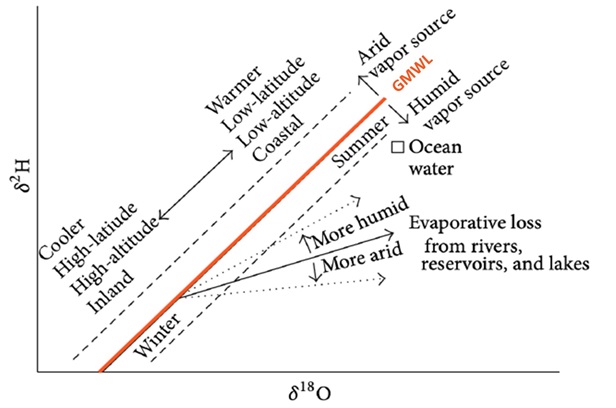
Figure 4. Factors influencing the distribution of water isotopic composition (SAHRA, 2005)
Isotopic fractionation leads to differences in stable isotope ratios (2H/1H and 18O/16O) and offers numerous possibilities for research within the hydrogeological cycle, such as defining the recharge areas, tracing the origin of water, determining the age of water, hydrodynamic conditions, rate of water exchange within aquifer and hydraulic connections between layers/aquifers. Since 18O and 2H are components of the water molecule, they are its ideal tracer.
Stable isotopes concentrations in water samples is usually determined by mass or laser spectrometers. The Laboratory for Spectroscopy, at the Faculty of Mining, Geology and Petroleum Engineering within University of Zagreb, concentrates on stable water isotope analyses. Stable isotopes, δ18O and δ2H in liquid water samples, are measured by absorption spectroscopy method using a „Laser Water Isotope Analyzer“ (LWIA-45-EP) from the „Los Gatos Research“ (LGR) (Figure 5). At this moment The Faculty of Mining, Geology and Petroleum Engineering participates in few scientific projects within which water stable isotopes are used. The most important are:
- SUPREHILL project (Subsurface preferential transport processes in agricultural hillslope soils) financed by Croatian Science Foundation – within this project the main aim is to quantify soil water subsurface preferential flow in agricultural hillslope soils;
- IAEA TC project CRO7002 (Using Nitrogen and Oxygen Stable Isotopes in the Determination of Nitrate Origin in the Unsaturated and Saturated Zone of the Velika Gorica Wellfield) financed by IAEA – the main goal of this project is to determine nitrate origin in the wider area of the Velika Gorica wellfield;
- IAEA TC project RER7013 (Evaluating Groundwater Resources and Groundwater-Surface-Water Interactions in the Context of Adapting to Climate Change) financed by IAEA – within this project numerous pilot areas have been established. One of the pilot areas is Sava River basin where relationship between precipitation, Sava River and alluvial aquifers will be defined using water stable isotopes;
- NATURAVITA project (Demining, restoration and protection of forest and forestland in protected and Natura 2000 sites in Danube-Drava regions) financed by the European Structural and Investment Funds, which presents a strategic project aimed at demining, reconstruction and protection of forest, forestland and water resources.

Figure 5. Laser spectrometer LWIA-45-EP (photo by Zoran Kovač)
References:
Clark I. (2015): Groundwater Geochemistry and Isotopes. CRC Press, Taylor & Francis Group, 438 p.
Mook, W. M. E. (2001): Environmental Isotopes in the Hydrological Cycle. Principles and Applications. UNESCO/IAEA Series.
SAHRA, Sustainability of semi-Arid Hydrology and Riparian Areas, Isotopes: Oxygen, Arizona, Ariz, USA, 2005
Xi X. (2014): A Review of Water Isotopes in Atmosferic General Circulation Models: Recent Advances and Future Prospects. International Journal of Atmosferic Sciences 2014, 1-16.
Patricia Buškulić, mag.ing.geol., is a Research Assistant at the Faculty of Mining, Geology and Petroleum Engineering within the University of Zagreb on IAEA TC project CRO7002 "Using Nitrogen and Oxygen Stable Isotopes in the Determination of Nitrate Origin in the Unsaturated and Saturated Zone of the Velika Gorica Wellfield".
E-portfolio https://moodle.srce.hr/eportfolio/user/view.php?id=17161
Google Scholar https://scholar.google.com/citations?user=g_kQ6foAAAAJ&hl=en



